Navigate FDA Medical Device Regulations with Confidence
Search 800+ CFR sections and 2,775+ FDA guidance documents in one place. Built for regulatory professionals, quality engineers, and medical device manufacturers who need answers fast.
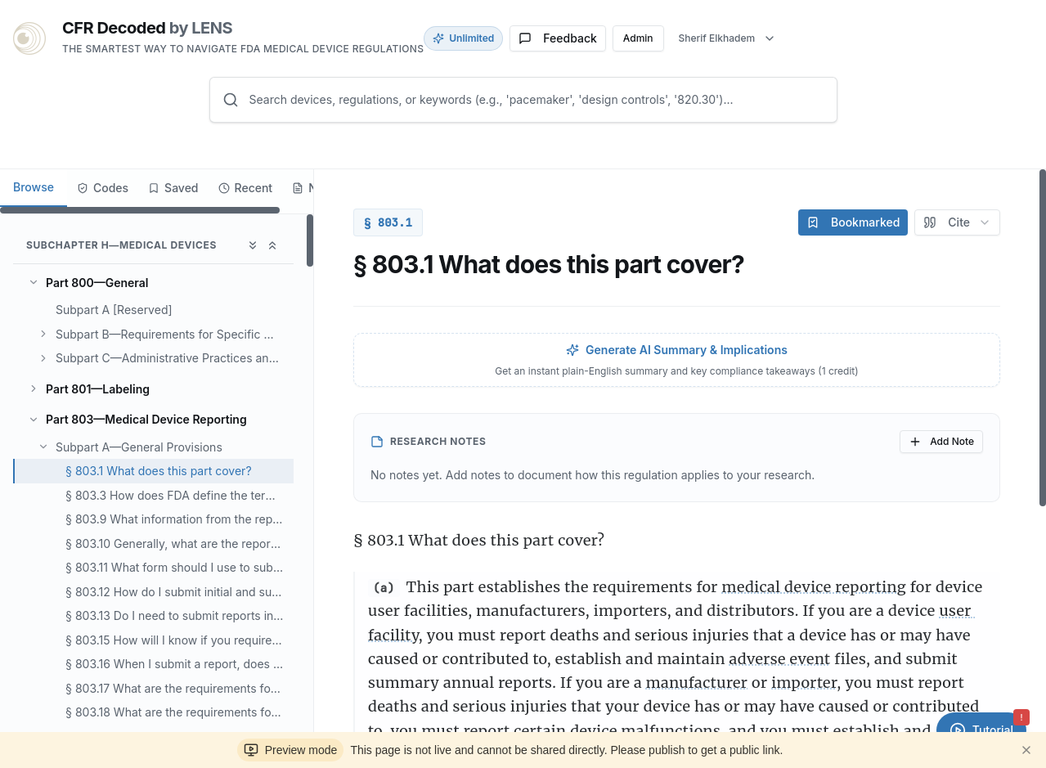
Intelligent Regulatory Tools
Everything you need to navigate FDA regulations efficiently
Comprehensive Search
Search across 800+ CFR sections and 2,775+ FDA guidance documents in one unified platform. Smart autocomplete and contextual results.
AI-Powered Assistant
Get instant plain-English summaries of complex regulations. Understand compliance implications in seconds.
Research Organization
Bookmark sections, create organized notes with folders, and build your regulatory knowledge base.
Credential Vault
Securely store FDA portal passwords with AES-256 encryption and AWS Secrets Manager.
QMSR Gap Analysis
Assess your QMS compliance with our guided wizard and generate detailed gap reports.
How It Works
Get started in three simple steps
Search or Browse
Use intelligent search to find specific requirements or browse the CFR hierarchy by Part and Section.
Understand with AI
Generate AI summaries to quickly grasp complex regulatory language and compliance requirements.
Save & Organize
Bookmark important sections, add notes with folders, and build a personalized regulatory reference library.
